The rapid diagnostic kits market has emerged as a critical segment in the global healthcare landscape, offering quick and efficient solutions for disease detection. These kits are indispensable for a range of conditions, from infectious diseases like COVID-19 and malaria to chronic illnesses like diabetes. However, while the market presents significant opportunities for growth, it also faces several challenges. This article delves into the key dynamics shaping the future of the rapid diagnostic kits market.
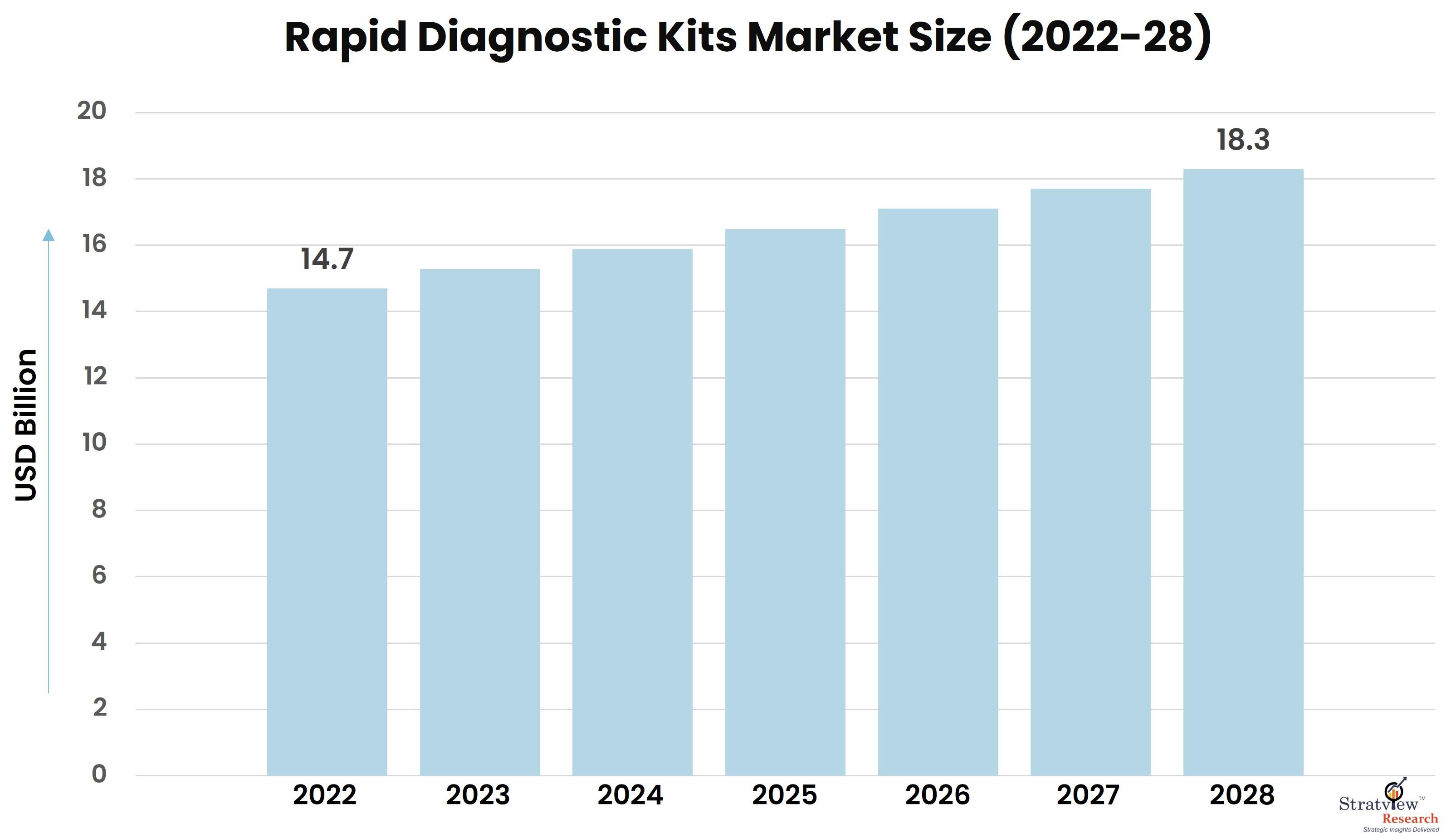
According to Stratview Research, the rapid diagnostic kits market was estimated at USD 14.7 billion in 2022 and is likely to grow at a CAGR of 3.66% during 2023-2028 to reach USD 18.3 billion in 2028.
Opportunities in the Rapid Diagnostic Kits Market
1. Increasing Demand for Point-of-Care Testing (POCT): One of the biggest opportunities for growth in the rapid diagnostic kits market is the increasing demand for point-of-care testing (POCT). With the rise of decentralized healthcare, patients and healthcare providers are opting for immediate, on-site diagnostic solutions that reduce the need for laboratory testing. Rapid diagnostic kits enable faster treatment decisions, improving patient outcomes, and are particularly useful in remote and underserved areas.
2. Growing Consumer Preference for Home Testing: The COVID-19 pandemic amplified the trend of home-based testing, with consumers increasingly seeking convenience in healthcare. From pregnancy tests to glucose monitoring and even at-home COVID-19 tests, the shift towards home testing represents a massive growth opportunity. The ability to manage personal health with rapid, easy-to-use diagnostic kits is transforming consumer behavior, encouraging manufacturers to develop user-friendly products with accurate results.
3. Rising Prevalence of Infectious and Chronic Diseases: The ongoing fight against infectious diseases such as COVID-19, HIV, and influenza continues to drive demand for rapid diagnostic kits. Additionally, the growing global burden of chronic diseases, including diabetes, cardiovascular diseases, and cancer, requires continuous monitoring and early detection—functions that rapid diagnostic kits can efficiently fulfill. This increasing prevalence offers significant market potential for manufacturers focusing on these conditions.
Challenges in the Rapid Diagnostic Kits Market
1. Regulatory Hurdles and Approval Processes: Despite the growing demand, the rapid diagnostic kits market faces regulatory challenges. Manufacturers must navigate complex approval processes and meet stringent standards set by agencies like the FDA or the European Medicines Agency (EMA). Delays in regulatory approvals can slow down the introduction of new products, impeding market expansion.
2. Accuracy and Reliability Concerns: While rapid diagnostic kits are convenient, concerns around accuracy and false results remain a significant challenge. False positives or negatives can lead to incorrect diagnoses, impacting patient care and eroding trust in these products. Improving accuracy and reliability through technological advancements will be essential for the market’s long-term success.
3. High Development and Manufacturing Costs: Developing high-quality rapid diagnostic kits often requires advanced technologies, driving up research, development, and production costs. These high costs can be a barrier to entry, particularly for smaller players in the market, and can result in expensive end products that may not be affordable for all consumers.
Conclusion
The rapid diagnostic kits market is poised for growth, driven by rising demand for point-of-care testing, home diagnostics, and the increasing prevalence of both infectious and chronic diseases. However, to fully capitalize on these opportunities, the industry must address challenges related to regulatory approvals, accuracy, and manufacturing costs. With the right strategies, the market can continue to play a pivotal role in modern healthcare, offering convenient and effective diagnostic solutions.