"Global Specimen Validity Testing Market – Industry Trends and Forecast to 2030
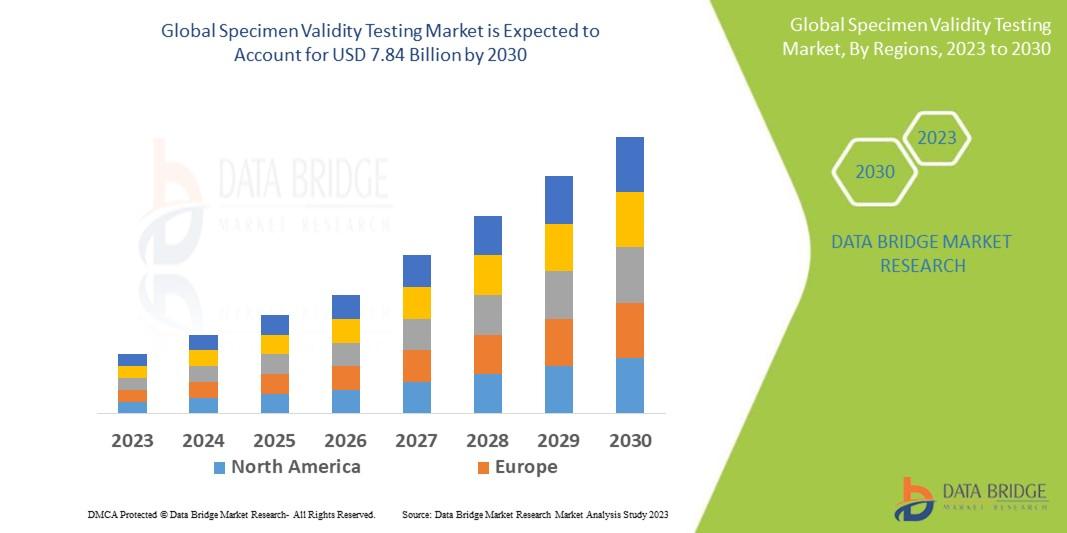
Data Bridge Market Research analyses that the specimen validity testing market which is USD 4.65 billion in 2022, is expected to reach USD 7.84 billion by 2030, at a CAGR of 6.75% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Access Full 350-page PDF Report @
https://www.databridgemarketresearch.com/reports/global-specimen-validity-testing-market
SVT is important in health care efforts because it measures patient adherence, morale, and dependability in consultation with clinicians. SVT is commonly treated by therapists, who use the results to make treatment decisions for their patients' medical problems, such as medication and illegal drug use. In the absence of SVT, the health care provider may miss the patient's urine sample deviation in an effort to deceive the provider.
**Segments**
- **Product Type**: The specimen validity testing market can be segmented based on product type into reagents and assay kits, analyzers, and other products. Reagents and assay kits are essential components in conducting specimen validity testing, while analyzers play a crucial role in automating and standardizing the testing process.
- **End-User**: The market can be segmented by end-user into hospitals, diagnostic laboratories, drug testing centers, and other end-users. Hospitals and diagnostic laboratories are key end-users of specimen validity testing products, utilizing them for various diagnostic and monitoring procedures.
- **Technology**: Specimen validity testing market segmentation based on technology includes immunoassay, chromatography, spectroscopy, and other technologies. Each technology offers unique advantages in terms of accuracy, sensitivity, and specificity in detecting specimen validity.
**Market Players**
- Abbott
- F. Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- Siemens Healthcare Private Limited
- Covidien plc
- Agilent Technologies
- Alere Inc.
- LabCorp
- QIAGEN
- NMS Labs
- American Screening LLC
To know more about the Specimen Validity Testing Market, visit https://www.databridgemarketresearch.com/reports/global-specimen-validity-testing-marketThe global specimen validity testing market is experiencing substantial growth and shows promising opportunities for market players. The increasing awareness regarding the importance of specimen validity testing in ensuring accurate drug test results is one of the primary factors driving market growth. With a rise in drug abuse cases worldwide, the demand for specimen validity testing solutions is escalating, particularly in hospitals, diagnostic laboratories, and drug testing centers. These end-users are crucial contributors to the market's growth as they rely on specimen validity testing products for precise diagnostic and monitoring procedures.
In terms of product type segmentation, reagents and assay kits play a pivotal role in specimen validity testing as they are fundamental components required for conducting accurate tests. Analyzers are also significant in automating and standardizing the testing process, thereby improving efficiency and reducing the margin of error. The market players mentioned, such as Abbott, Thermo Fisher Scientific Inc., and Siemens Healthcare Private Limited, offer a range of products, including reagents, assay kits, and analyzers, catering to the diverse needs of end-users in the specimen validity testing market.
Technology segmentation in the specimen validity testing market showcases a variety of options such as immunoassay, chromatography, spectroscopy, and other innovative technologies. Each technology brings its unique advantages in terms of accuracy, sensitivity, and specificity in detecting specimen validity, thereby enhancing the overall testing process. Companies like F. Hoffmann-La Roche Ltd, Alere Inc., and QIAGEN are at the forefront of incorporating advanced technologies into their specimen validity testing products, aligning with the market's growing demand for precise and reliable testing solutions.
Moreover, market players like Covidien plc, Agilent Technologies, and LabCorp are consistently focusing on research and development activities to introduce new and improved specimen validity testing products in the market. This strategy not only helps them stay competitive but also enables them to meet the evolving requirements of end-users across different segments. Collaborations, partnerships, and strategic acquisitions are also common among market players to strengthen their market presence and expand**Segments**
- **Product Type**: The specimen validity testing market is segmented into reagents and assay kits, analyzers, and other products. Reagents and assay kits are crucial components for conducting tests, while analyzers automate and standardize the testing process.
- **End-User**: End-users include hospitals, diagnostic laboratories, drug testing centers, and others. Hospitals and diagnostic labs rely on these products for diagnostic and monitoring procedures.
- **Technology**: The market is segmented by technology into immunoassay, chromatography, spectroscopy, and others, each offering unique advantages in detecting specimen validity.
**Market Players**
- Abbott
- F. Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- Siemens Healthcare Private Limited
- Covidien plc
- Agilent Technologies
- Alere Inc.
- LabCorp
- QIAGEN
- NMS Labs
- American Screening LLC
The global specimen validity testing market is witnessing robust growth due to the increasing awareness of the importance of accurate drug test results. The surge in drug abuse cases globally has heightened the demand for specimen validity testing solutions, particularly in hospitals, diagnostic laboratories, and drug testing centers. These end-users are vital contributors to market growth as they rely on these products for precise diagnostic procedures. Reagents and assay kits are essential for accurate testing, while analyzers streamline the process, reducing errors. Market leaders like Abbott and Thermo Fisher Scientific Inc. offer a range of products satisfying the
Core Objective of Specimen Validity Testing Market:
Every firm in the Specimen Validity Testing Market has objectives but this market research report focus on the crucial objectives, so you can analysis about competition, future market, new products, and informative data that can raise your sales volume exponentially.
- Size of the Specimen Validity Testing Market and growth rate factors.
- Important changes in the future Specimen Validity Testing Market.
- Top worldwide competitors of the Market.
- Scope and product outlook of Specimen Validity Testing Market.
- Developing regions with potential growth in the future.
- Tough Challenges and risk faced in Market.
- Global Specimen Validity Testing top manufacturers profile and sales statistics.
Key takeaways from the Specimen Validity Testing Market report:
- Detailed considerate of Specimen Validity Testing Market-particular drivers, Trends, constraints, Restraints, Opportunities and major micro markets.
- Comprehensive valuation of all prospects and threat in the
- In depth study of industry strategies for growth of the Specimen Validity Testing Market-leading players.
- Specimen Validity Testing Market latest innovations and major procedures.
- Favorable dip inside Vigorous high-tech and market latest trends remarkable the Market.
- Conclusive study about the growth conspiracy of Specimen Validity Testing Market for forthcoming years.
Frequently Asked Questions
- What is the Future Market Value for Specimen Validity Testing Market?
- What is the Growth Rate of the Specimen Validity Testing Market?
- What are the Major Companies Operating in the Specimen Validity Testing Market?
- Which Countries Data is covered in the Specimen Validity Testing Market?
- What are the Main Data Pointers Covered in Specimen Validity Testing Market Report?
Browse Trending Reports:
Mice Model Market
Coffee And Tea Manufacturing Market
Pastry Fillings Market
Injection Devices For Biological Drugs Market
Bath Salts Market
Storyboarding Software Market
Casino Online Gambling Market
Fortified Dairy Products Market
Blockchain In Agriculture And Food Supply Chain Market
Stand Up Paddleboard Market
Natural Food Colors And Flavors Market
Grapeseed Oil Market
Supplier Oriented Business To Business e Commerce Market
Household Water Softener System Market
Trace Minerals In Poultry Feed Market
Dissolving Wood Pulp Market
Blockchain Insuretech Market
Activated Carbon Filters Market
Omega 21 Market
Flight Simulator Market
Dashboard Camera Market
Companion Animal Diagnostic Market
Next Generation Tobacco Products Market
Commercial Air Filter Market
Fruit And Vegetable Powders Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975