"Clinical Trial Supplies Market – Industry Trends and Forecast to 2031
Global Clinical Trial Supplies Market, By Services (Storage, Manufacturing, Packaging and Labelling and Distribution), Clinical Phase (Phase III, Phase II, Phase IV, Phase I), Therapeutic Uses (Oncology, Cardiovascular Diseases, Dermatology, Metabolic Disorders, Infectious Diseases, Respiratory Diseases, CNS and Mental Disorders, Blood Disorders, Others), End User (Contract Research Organizations, Pharmaceutical and Biotechnology Companies) – Industry Trends and Forecast to 2031.
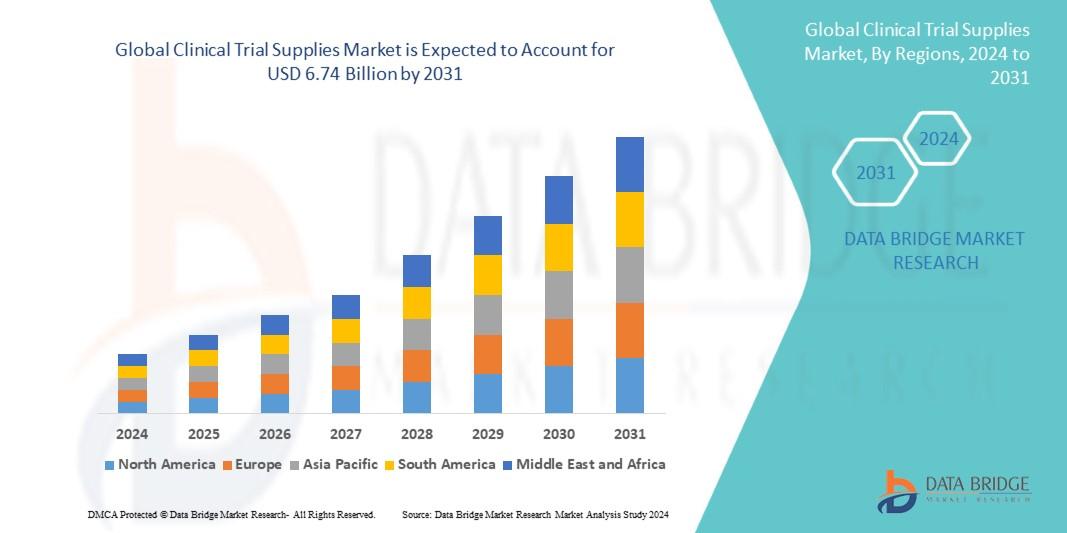
Data Bridge Market Research analyses that the global clinical trial supplies market, which was USD 3.53 billion in 2023, is expected to reach USD 6.74 billion by 2031, and is expected to undergo a CAGR of 8.4% during the forecast period of 2024 to 2031. This indicates that the market value. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Access Full 350 Pages PDF Report @
https://www.databridgemarketresearch.com/reports/global-clinical-trial-supplies-market
Clinical trial supplies refer to the materials and equipment essential for conducting medical research on new treatments or interventions. These supplies encompass medications, placebos, medical devices, and ancillary items required for the trial's execution. Ensuring timely and accurate provision of these resources is critical for the integrity and success of the trial.
**Segments**
- The Clinical Trial Supplies Market can be segmented on the basis of product type, stage, and end user. Product type segments include manufacturing supplies, packaging and labeling materials, and clinical trial equipment. Manufacturing supplies consist of active pharmaceutical ingredients (API), excipients, and others necessary for drug production. Packaging and labeling materials encompass vials, blisters, bottles, labels, and other materials required for secure packaging. Clinical trial equipment covers a wide range of tools such as centrifuges, chromatography systems, and refrigerators used in research settings. The stage of the trial can be categorized into phase I, phase II, phase III, and phase IV, where the complexity and scale of supplies required increase with each phase. End user segments include pharmaceutical companies, contract research organizations (CROs), research institutes, and others involved in clinical trials.
**Market Players**
- The Clinical Trial Supplies Market comprises several key players who play crucial roles in ensuring the smooth running of trials. Some prominent market players include Catalent, Inc., Parexel International Corporation, Thermo Fisher Scientific Inc., Almac Group, PAREXEL International Corporation, PCI Pharma Services, Sharp Clinical Services, Movianto, Biocair, and CSM. These companies offer a diverse range of services such as packaging, labeling, storage, and logistics for clinical trial supplies. They work closely with pharmaceutical companies, research organizations, and other stakeholders to provide efficient and reliable solutions throughout the trial process.
https://www.databridgemarketresearch.com/reports/global-clinical-trial-supplies-marketThe Clinical Trial Supplies Market is experiencing steady growth due to the increasing demand for efficient and reliable solutions in the pharmaceutical industry. One key trend that is shaping the market is the rising focus on personalized medicine, driving the need for specialized clinical trial supplies tailored to individual patient needs. This trend is prompting market players to innovate and develop customized products and services to cater to this niche segment.
Another significant factor driving market growth is the growing outsourcing trend in the pharmaceutical sector. Pharmaceutical companies are increasingly partnering with contract research organizations (CROs) to conduct clinical trials, leading to a surge in demand for outsourced clinical trial supplies services. This outsourcing trend is expected to continue to drive market growth as companies seek to streamline operations and reduce costs.
The market is also witnessing a shift towards virtual clinical trials, especially in the wake of the COVID-19 pandemic. Virtual trials offer several advantages, including reduced patient burden, increased patient enrollment, and cost savings. As a result, there is a growing demand for innovative clinical trial supplies that can support virtual trial operations, such as remote monitoring devices, ePRO solutions, and direct-to-patient services.
Moreover, regulatory changes and stringent quality standards are playing a crucial role in shaping the Clinical Trial Supplies Market. With regulatory authorities imposing strict guidelines on the handling and storage of clinical trial supplies, market players are focusing on compliance and quality assurance to ensure the safety and integrity of the trial process. This emphasis on regulatory compliance is driving investments in advanced technologies and quality management systems to meet the evolving requirements of the industry.
In terms of competitive landscape, key players in the Clinical Trial Supplies Market are continuously investing in research and development to launch innovative products and services. Companies are also expanding their geographic presence through strategic partnerships and acquisitions to strengthen their market position. Additionally, collaborations between industry players and academic institutions are fostering innovation and driving market growth through knowledge sharing and resource pooling.
Looking ahead, the Clinical Trial Supplies Market is poised for significant growth driven by technological advancements, increasing outsourcing trends, and evolving regulatory**Global Clinical Trial Supplies Market Analysis**
The Global Clinical Trial Supplies Market is witnessing significant growth driven by various factors such as the increasing demand for personalized medicine, growing outsourcing trends, and the adoption of virtual clinical trials. The market is segmented based on services, clinical phase, therapeutic uses, and end user. Under services, key segments include storage, manufacturing, packaging and labeling, and distribution. Clinical phase segmentation comprises Phase III, Phase II, Phase IV, and Phase I, reflecting the progression of trial stages. Therapeutic uses categories encompass Oncology, Cardiovascular Diseases, Dermatology, Metabolic Disorders, Infectious Diseases, Respiratory Diseases, CNS and Mental Disorders, Blood Disorders, and Others. End users are Contract Research Organizations, Pharmaceutical and Biotechnology Companies.
Market players in the Clinical Trial Supplies Market continue to invest in research and development to introduce innovative products and services. Companies are expanding their geographical footprint through strategic partnerships and acquisitions to enhance their market presence. Furthermore, collaborations between industry players and academic institutions are driving innovation and market growth through knowledge exchange and resource pooling.
The increasing focus on personalized medicine is a key trend shaping the Clinical Trial Supplies Market. As the demand for specialized clinical trial supplies tailored to individual patient needs rises, market players are innovating to develop customized solutions for this niche segment. Additionally, the growing outsourcing trend in the pharmaceutical sector, with companies partnering with CROs for clinical trials, is fueling demand for outsourced clinical trial supplies services. This trend is expected to sustain
Countries Studied:
- North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
- Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
- Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
- Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Key Coverage in the Clinical Trial Supplies Market Report:
- Detailed analysis of Clinical Trial Supplies Market by a thorough assessment of the technology, product type, application, and other key segments of the report
- Qualitative and quantitative analysis of the market along with CAGR calculation for the forecast period
- Investigative study of the market dynamics including drivers, opportunities, restraints, and limitations that can influence the market growth
- Comprehensive analysis of the regions of the Clinical Trial Supplies industry and their futuristic growth outlook
- Competitive landscape benchmarking with key coverage of company profiles, product portfolio, and business expansion strategies
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse Trending Reports:
Wire And Cable Market
Teeth Whitening Light Market
Compound Feeds And Additives Market
Scara Robot Market
Child Resistant Packaging Market
Automotive Digital Cockpit Market
Ammonium Sulfate Market
Rodenticides Market
Automotive Safety System Market
Barbeque Sauce Market
Acute Lymphocytic Lymphoblastic Leukemia All Therapeutics Market
Patient Temperature Management Market
Chemical Vapor Deposition Cvd Equipment Market
Hvac Air Quality Monitoring Market
In Vehicle Infotainment Market
Mycoplasma Testing In Clinical Market
Phosphorescent Pigments Market
Ankylosing Spondylitis As Market
Dental Light Curing Equipment Market
Train Signalling System Market
Oral Irrigator Market
Sepsis Disease Treatment Market
Pediatric Genetic Disease Treatment Market
Ipm Pheromones Market
Variable Frequency Drive Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975